About
About this Poster:
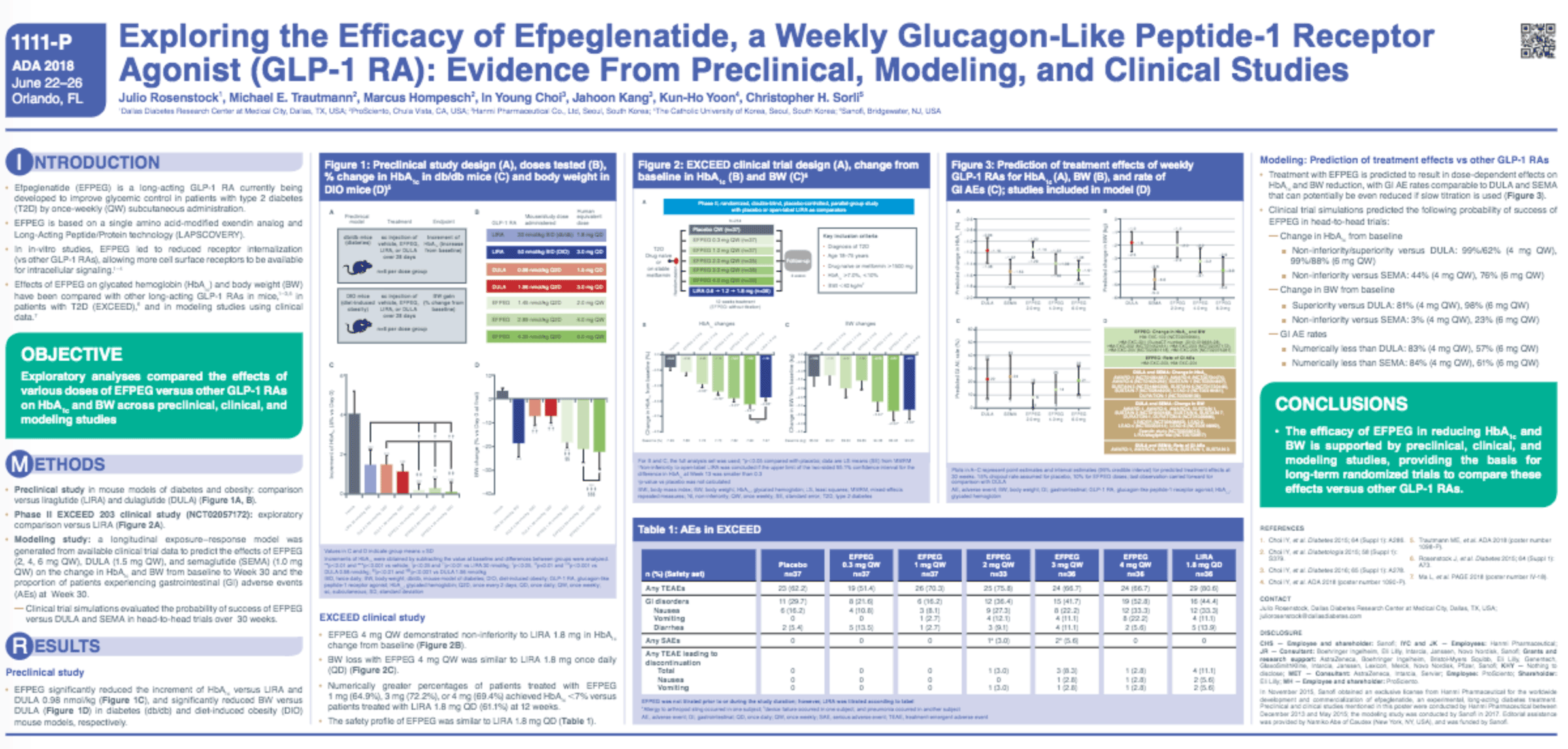
Once-weekly (QW), subcutaneous efpeglenatide (efpeg) is a long-acting GLP-1 RA in development for type 2 diabetes (T2D). In vitro studies have shown that efpeg is a superagonist: an agent that can achieve a greater maximal effect than that of the endogenous ligand. Exploratory analyses of preclinical, modeling, and clinical data investigated the effects of efpeg on glycated hemoglobin (HbA1c) and body weight (BW) vs. dulaglutide (dula), liraglutide (lira), and semaglutide (sema) (Table). In preclinical studies, efpeg significantly reduced the increment of HbA1c vs. lira and dula in db/db mice, and reduced BW vs. dula in diet-induced obesity mice. Predictive treatment effects of GLP-1 RAs over 30 weeks (based on modeled data) suggest that efpeg 6 mg QW has a high probability of superiority vs. dula on HbA1c and BW, and similar effects on HbA1c vs. sema; 4 mg QW has a high probability of non-inferiority and superiority vs. dula on HbA1c and BW endpoints, respectively. Analyses of the EXCEED 203 dose-ranging study in T2D suggest that efpeg 4 mg QW is at least non-inferior to lira on HbA1c reduction; safety profiles and effects on BW were comparable. In conclusion, the efficacy of efpeg in reducing HbA1c and BW is supported by preclinical, modeling, and clinical studies, providing the basis for long-term randomized trials to compare these effects vs. other GLP-1 RAs.