Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in North America, and nonalcoholic steatohepatitis (NASH) is a growing contributor to liver transplantation and liver-related mortality rates. The relatively asymptomatic progression of NAFLD and NASH presents challenges in patient and physician awareness, combined with gaps in knowledge regarding pathogenic drivers, adds to the complexities of NASH clinical drug development. There is an urgent need to develop noninvasive tools for diagnosis and disease tracking, as well as therapeutic strategies, for patients with NASH, from early stages through advanced liver fibrosis. This webinar will address these unique challenges by defining strategies for the design and enrollment of clinical studies, as well as the study of disease progression and biomarker discovery.

Recommended Materials

Posters
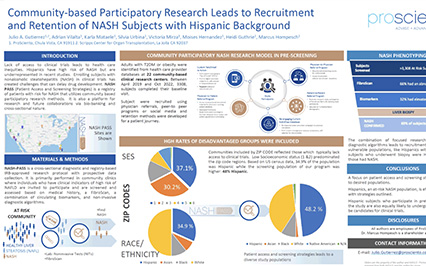
NASH PASS®: A Registry To Inform Study Design And Accelerate Patient Enrollment